1. What is pH?
The pH is a measure of how acid or basic the water is. The pH measures the concentration of hydrogen ions (H+) in the water. The actual definition of pH is the negative log of the hydrogen ion concentration. The pH scale ranges from 0-14 and a pH of 7 is neutral. A pH below 7 is acidic and a pH above 7 is basic (see the graphic scale below). Because values of pH are based on a logarithmic scale, each 1.0 change in pH represents a factor of ten change in acidity. This means that a pH of 3.0 is 10 times more acidic than a pH of 4.0.
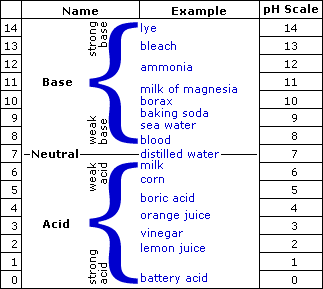
The average pH of seawater is around 8.2, slightly basic. During photosynthesis, hydrogen atoms are used by phytoplankton and the pH will rise, becoming more basic. Respiration and the breakdown of organic matter will lower the pH, making the water more acidic. In seawater, the pH doesn't change very much because seawater has a natural buffer. A buffer prevents the pH from changing because chemical reactions occur to balance changes in the concentration of hydrogen ions.
Most organisms living in estuaries prefer a pH between 6.5 and 8.5. If the pH drops below 5.0 or goes above 9.0, many marine organisms will have trouble surviving.
| 


