1. What is dissolved oxygen?
Dissolved oxygen (DO) is the amount of oxygen (O2) dissolved in the water. Dissolved oxygen is one of the best indicators of water quality. People need oxygen in the atmosphere to survive and animals that live in the ocean, like fish, need dissolved oxygen in the water to survive.
The amount of dissolved oxygen that the water can hold depends on the temperature and salinity of the water. Cold water can hold more dissolved oxygen than warm water and fresh water can hold more dissolved oxygen than salt water. So the warmer and saltier the water, the less dissolved oxygen there can be. The maximum amount of dissolved oxygen that the water can hold is called the saturation value. Dissolved oxygen measurements are given as a percent of saturation (%) or in units of milligrams per liter (mg/l).
Oxygen enters the water at the surface of the water where exchange between the atmosphere and the water can take place. Waves and wind help put oxygen into the water. Dissolved oxygen is also put into the water as a byproduct of phytoplankton photosynthesis. The oxygen found in the deeper water comes from mixing with surface water. Photosynthesis can cause the water to have more dissolved oxygen than the saturation amount. When that happens it is called supersaturation.
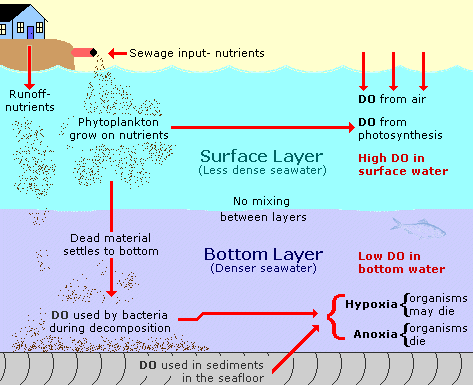
Animals, such as fish, breathing in the water consume dissolved oxygen. It is also used in the break down of organic matter. As organic matter sinks to the sea floor it begins to decompose. Bacteria in the water use oxygen to break down this organic material. When there is a lot of organic debris, the dissolved oxygen in the deeper water can be used up. If the water at the surface (which has plenty of dissolved oxygen) is not mixed with the deeper water layers form and the water becomes stratified. Then there is no new dissolved oxygen for the deep water. When this happens, the deep water can become unhealthy.
Above 5 mg/l dissolved O2, most marine plants and animals have plenty of oxygen. When the dissolved oxygen is low, below 3 mg/l, the water is called hypoxic. If all the dissolved oxygen is used up, below 0.5 mg/l, the water is called anoxic. Under hypoxic conditions, many marine plants and animals may not survive. No marine plants and animals that require oxygen can survive in anoxic conditions.
Approximate dissolved oxygen saturation values
(At a salinity of 30ppt):
Temperature
(°C) |
 |
Dissolved
oxygen (mg/l) |
 |
| 30 |
 |
6.4 |
 |
| 25 |
 |
7.0 |
 |
| 20 |
 |
7.6 |
 |
| 15 |
 |
8.4 |
 |
| 10 |
 |
9.3 |
 |
| 5 |
 |
10.5 |
 |
| 


