1. What is Salinity?
Salinity is the measure of all the salts dissolved in water. Salinity is usually measured in parts per thousand (ppt or 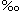 ). The average ocean salinity is 35ppt and the average river water salinity is 0.5ppt or less. This means that in every kilogram (1000 grams) of seawater, 35 grams are salt. Because the water in estuaries is a mix of fresh water and ocean water, the salinity in most estuaries is less than the open ocean. Bottom water almost always contains more salt than surface waters. ). The average ocean salinity is 35ppt and the average river water salinity is 0.5ppt or less. This means that in every kilogram (1000 grams) of seawater, 35 grams are salt. Because the water in estuaries is a mix of fresh water and ocean water, the salinity in most estuaries is less than the open ocean. Bottom water almost always contains more salt than surface waters.
The salt in the ocean is mostly made up of the elements sodium (Na) and chlorine (Cl). Together they account for 85.7% of the dissolved salt. The other major components of seawater are magnesium (Mg), calcium (Ca), potassium (K) and sulfate (SO4). Together with chlorine and sodium they make up 99.4% of the salt in the ocean.
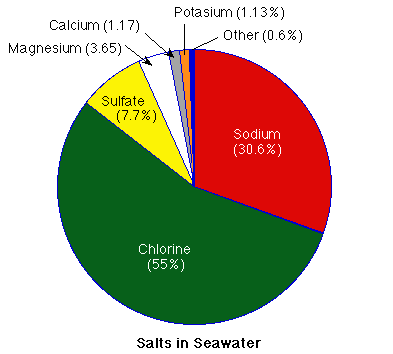
What else is dissolved in the seawater?
Does table salt come from the ocean?
Why does salt water taste bitter?
| 


